Aerogels: What Material Is Lighter Than a Feather?
- katerinabiryukova
- May 25
- 4 min read
Updated: Jul 14
Have you ever wondered what allows space machinery to be insulated from the extremely high and low temperatures that it is subjected to as it traverses through space?
The answer to this question is a material with the peculiar name “aerogel”, also nicknamed “frozen smoke”. This is a very porous substance (in fact, over 90% of an aerogel’s content is air) that is also the most lightweight material in the world. By appearance, it is almost transparent, having a blue tint. Surprisingly, aerogels were first invented about 25 years before the start of the Space Race and were incited by the most unexpected object – a jelly.
Structure of a Gel
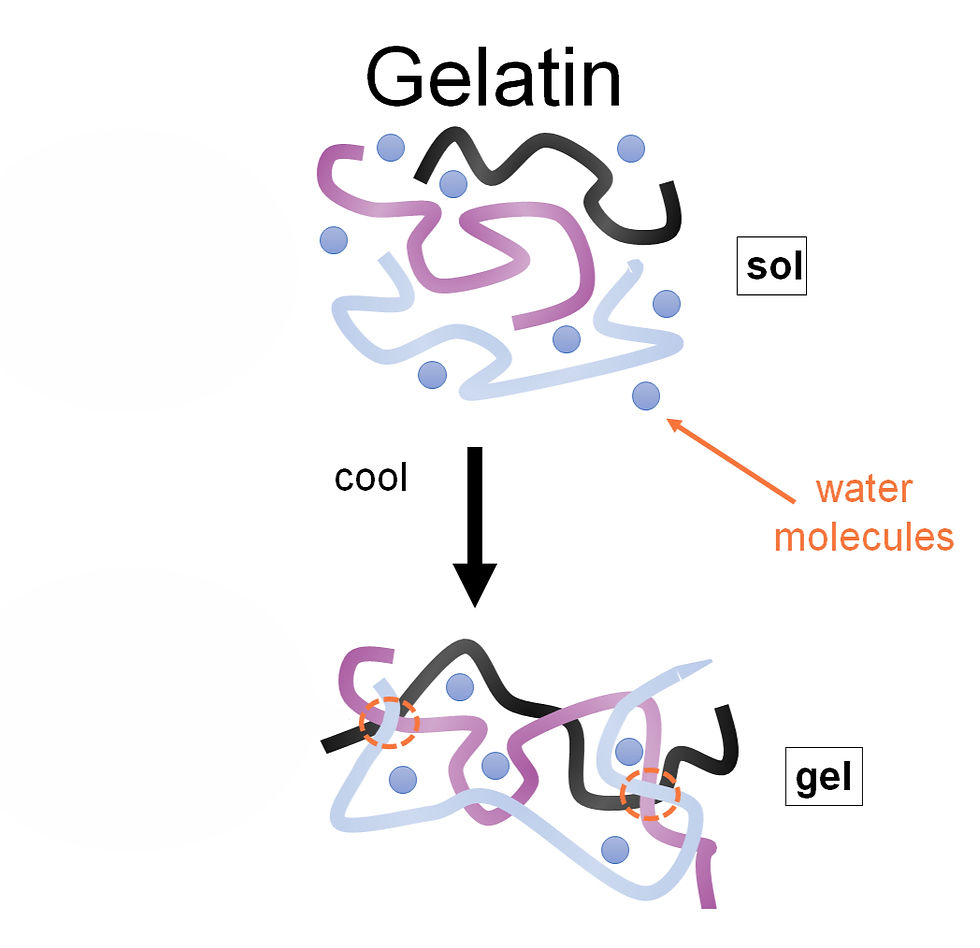
In 1931, a chemist named Samuel Kistler made a bet with his fellow colleague as to whether he would be able to replace the liquid inside a jelly jar without causing the main structure to shrink. Now, a jelly is comprised of water and long chains of gelatin molecules, which span the entire jelly and trap the liquid within. The water is held inside by surface tension – the force due to the cohesion (attraction to one another) of water molecules. In a jelly, the surface tension forces that are strong enough to prevent the liquid from escaping the jelly, but weak enough, so that a jelly can slosh around. Interestingly, surface tension is also responsible for formation of water drops.
Creating Aerogels
The method of synthesizing an aerogel is as follows: first, the “sol-gel” process is used. A free-flowing liquid colloidal solution (a sol), is converted to a solid 3D network of molecules surrounding the liquid (a gel) - we have seen this in cooling water added to gelatine to create a jelly. Next, the gel is aged to increase its strength.
After that, the liquid is carefully removed from the gel using the superficial drying process: the temperature and pressure of the gel is increased above the critical point. The pressure is then reduced, so the liquid inside vaporizes and is removed, leaving an aerogel.
An extension to superficial drying, developed by Dr. Arlon Hunt at Lawrence National Laboratory in 1980’s, involves the use of liquid carbon dioxide. After the gel is formed, it is left to soak in this liquid, which is at high (~58 atmospheric) pressure because that is the only condition at which carbon dioxide can exist in the liquid state. After a period of time (1-14 days), the carbon dioxide replaces the initial liquid inside the gel, and the superficial drying process is then used to remove the CO2 and create an aerogel.
Additionally, freeze-drying has been utilized as an alternative to superficial drying.
Varying Aerogels
Aerogels are made of a variety of different materials, with silica aerogels being produced the most, followed by carbon and metal oxide aerogels. As an interesting fact, Kistler created aerogels not only from gelatine, but also aluminum, nickel tartrate, stannic oxide, nitrocellulose, cellulose, and egg albumin.
Properties of Aerogels
Aerogels possess some uncommon solid material characteristics, including a high surface area, very low density, strength and low thermal conductivity. This material is so good as a thermal insulator that when putting a Bunsen burner on one side of a piece of an aerogel and a flower on the other, the flower will still be conserved after a couple of minutes.


Applications
Aerogels’ properties have made them critical for many innovations:
• In 1997, NASA used silica aerogel in the Mars Pathfinder mission and has been using it ever since for spacecraft insulation. (This is due to the excellent thermal insulation and extremely light weight of the material.)
• In addition, NASA employed aerogel as a dust collector for the Stardust spacecraft (1999-2006).
• Aerogels can efficiently absorb toxic and harmful substances (contaminants) from wastewater and air, reducing pollution and slowing down climate change.
• Since 2020, aerogels have been increasingly used for fire protection of electrical vehicles’ batteries, both for preventing accidents and providing a longer escape time to the passengers.
• In the future, aerogels could be used for effective building insulation, including roof, floor, framing and even windows (as they are optically transparent). This could significantly lower energy use and cost by reducing the need of heating and cooling the indoor air.
• And much more!
Innovations
Furthermore, advances in the field of aerogels are constantly being made. Namely, NASA has developed polymer-enforced aerogels. A thin polymer layer is added to the interior surface of an aerogel, which greatly increases its strength and makes it less brittle. An even more promising innovation is creating aerogels completely out of polymers, so that they become extremely strong and flexible and can be bent into a thin film.

Resources
https://edu.rsc.org/everyday-chemistry/why-does-jelly-wobble/4015724.article
https://www.aerogel.org/?p=3#:~:text=An%20aerogel%20is%20an%20open,to%2050%20nm%20in%20diameter.
https://www.nasa.gov/aeronautics/aerogels-thinner-lighter-stronger/
https://www.aps.org/apsnews/2021/05/publication-creation-first-aerogel
https://www.idtechex.com/en/research-report/aerogels-2025-2035-technology-market-forecasts/1076
https://www.sciencedirect.com/science/article/abs/pii/S1385894716305484


Commentaires