Why Is Glass Transparent?
- katerinabiryukova
- Jun 18, 2025
- 5 min read
Updated: Aug 16, 2025
Each year 180 million tons of glass are produced, and practically every country has a glass industry (1).

We can see the work of this material all around us: in car windows, bottles, mirrors, light bulbs and cell phone touchscreens. Two of the applications of glass have even inherited its name: the container that holds a liquid while drinking (a glass) and the eyeglasses (often shortened to glasses).
What are the properties of glass that lead its ubiquitous use, and what is the science behind these properties? Let’s find out!
Amorphous solid
Even though glass is one of the oldest materials fabricated by humans (5), its composition is not as transparent as this material tends to be.
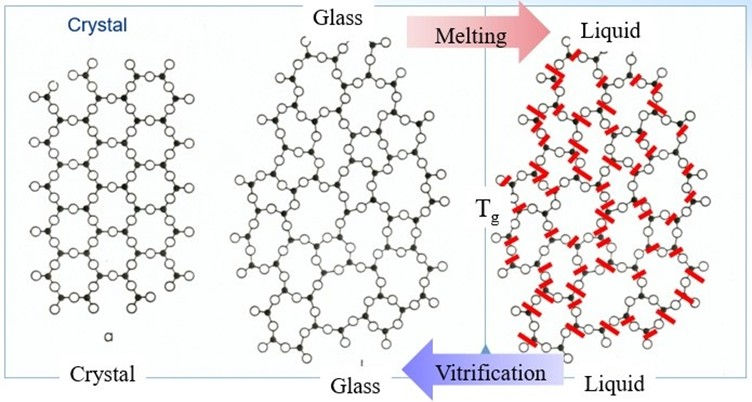
Glass is an amorphous solid. In a crystalline solid (this is what people generally refer to when they refer to the term ‘solid’), the particles are held in fixed positions in an ordered arrangement; meanwhile, in glass the particles are topologically disordered. To obtain such configuration, a raw material is melted and then quenched (rapidly cooled down) to avoid crystallization since atoms will return to their regular positions if given sufficient time.
Supercooled Liquid & Glass Transition Temperature
The transition of molten glass into its traditional solid state can roughly be divided into three stages:
Liquid: In this phase, the molecules inside the glass have no regular pattern and are moving freely (sliding past each other), which results in the liquid’s ability to flow. Furthermore, structurally, a liquid is comprised of microscopical percolation structures – networks of bonds between small clusters of atoms.
Supercooled Liquid: As molten glass is cooled down, its particles start to move slower because they have less kinetic energy (energy due to motion). However, before it fully solidifies, glass spends some time in the state of a supercooled liquid. This word refers to a liquid that remains liquid below its freezing point (the temperature at which, under normal conditions, a substance becomes a solid) and retains the liquid’s characteristics: the movement of the molecules and fluidity.
Amorphous Solid: When the supercooled liquid is cooled down further, at a certain point its particles stop flowing freely, and the substance becomes very “sticky” or viscous (at least 10^12 Pa*s). This is called the glass transition temperatures, Tg , and is plural because it is a range of temperatures rather than a single number (see more in section “Glass Properties”). Below Tg the glass exits as a solid – its particles are “frozen” in place and can only vibrate around their fixed positions. Compared to a liquid, the broken bonds in an amorphous solid are only point defects (meaning localized disruptions in the arrangement of atoms), unlike numerous broken bonds in the liquid’s percolations (2).
Glass Properties
This structure gives glass a couple of curious properties.
IFirstly, it is isotropic, meaning that its properties (such as refractive index, thermal expansion, or strength) are the same in all directions. The disordered bonding of glass holds responsibility for this characteristic. Contrastingly, in crystalline solids, the ordered bonding is usually focused in a specific direction, and they are anisotropic, having different values of the same property, when measured in a different direction.
Secondly, glass does not have a fixed melting point, but becomes liquid over a range of temperatures, typically 1400 to 1600 degrees C. This is because different atoms have varying strengths of chemical bonds (due to varying distances to their neighbor atoms and the number of those neighbors), leading to different amounts of thermal energy to overcome them. The melting point of glass also depends on its specific chemical composition.
Moreover, glass breaks unevenly, in a curved manner with slightly concave surfaces. This is called conchoidal fracture and occurs because there is no definite composition. When stress is applied, it doesn’t align with a particular plain and causes fracture in an unpredicted way.
Other notable features of glass include its strength, brittleness and transparency (3).
The Most Disturbing Question: Why is Glass Transparent?

As an introduction, a nucleus, which contains protons and neutrons, is located in the center of every atom, and electrons surround this nucleus. They move along certain restricted orbits, also called energy levels.
The light that is visible to our eye is a part of the electromagnetic spectrum, displaying wave-like and particle-like properties.
Wave-particle duality is a concept in quantum mechanics, stating that all quantum entities, eg. photons and electrons, exhibit wave-like and particle-like properties.
In this instance, it is more useful to consider the visible light as the particles called photons. Photons are not particles of matter, but packets of varying amounts of energies.
When light, or photons, passes through a material, one of the following situations occurs:
“The substance absorbs a photon”: The photon hands its energy over to an electron, which uses this energy to move to a higher energy level.
The photon is reflected: The photon yields its energy to the material, but then a photon with the same amount of energy gets emitted.
Transmission: The photon doesn’t interact with any electrons and passes through until it encounters another object.
At interacting with glass, photons display the last behavior. The reason behind this is that photons do not have a sufficient amount of energy to bump the electrons to a higher energy level. Sometimes referred to as band theory, there are band gaps between the energy levels, where electrons do not exist at all. While in most other materials these band gaps are small, in glass they are very large, and the photons of visible light (with 400-700 nanometers (nm) wavelengths) do not have the minimum amount of energy that an electron needs to jump between these gaps.

Curiously, photons with smaller wavelengths, such as ultraviolet light (also a part of the electromagnetic spectrum) with 10 to 400 nm wavelength and higher frequencies, have enough energy. Upon encountering glass, the UV photons exhibit the first two behaviors, mostly the absorption and some reflection. Thus, glass is generally opaque to ultraviolet light.
Nevertheless, some types of ultraviolet light are transmitted by glass. This is the UV-A class, which has longer wavelengths that the other types of UV light and is, hence, closer to the visible light on the electromagnetic spectrum (6).
Unexpected Application of Science
An example of this phenomena had once occurred with me in real life. When I was about 10 years old, I had a pair of trainers which could change their color from white to pink under the sun – when exposed to ultraviolet light. One time, I was sitting in a café next to the window while wearing these sneakers and observed an interesting effect. The ray of sun could clearly be seen passing through the glass and hitting my shoes; however, they were as white as a cloud on a stormless day. The visible light could be observed because it travelled freely through the glass, but the color of the trainers didn’t change as the UV photons were absorbed by the glass’s electrons.
Conclusion
In closing, the disordered structure of glass as an amorphous solid gives rise to its isotropy, lack of distinct melting point, conchoidal fracture, strenght and brittleness. The large gaps between energy levels in the atoms of glass are responsible for its transparency.
Bibliography
Author/AutorDr.-Ing. Joachim Harder, OneStone Consulting S.L., Barcelona/Spanien. (2019, December 10). Glass recycling – Current market trends. Recovery. https://www.recovery-worldwide.com/en/artikel/glass-recycling-current-market-trends-3248774.html#:~:text=Worldwide%2C%20around%20130%20million%20tonnes,flat%20glass%20is%20only%2011%20%25.
Ojovan, M. (2024, May 9). Materials science, glasses. https://encyclopedia.pub/entry/8713
GeeksforGeeks. (2024, March 5). Amorphous solid. GeeksforGeeks. https://www.geeksforgeeks.org/chemistry/amorphous-solid/
Harris, W. (2021, April 15). What makes glass transparent? HowStuffWorks. https://science.howstuffworks.com/question404.htm#:~:text=This%20is%20because%20of%20the,crystallized%20structure%2C%20thus%20causing%20transparency.
Human history: a tale of glass. (2022, June 23). LICHTGEDANKEN. https://www.lichtgedanken.uni-jena.de/en/1408/human-history-a-tale-of-glass
Does solar UV penetrate window glass. (2025, May 7). HPS. https://hps.org/publicinformation/ate/q12082/#:~:text=According%20to%20the%20International%20Ultraviolet%20Association%2C%20standard,only%20by%20specialized%20lights%20and%20the%20sun



Comments